Teratogenecity & Toxicity Unit
|
| Home >> Research Service Units >>
Teratogenecity & Toxicity Unit |
 |
|
Teratogenecity & Toxicity Unit
|
|
|
Coordinator :
Dr Muhammad Abu-Elmagd
Assistant :
Dr Mohammed Alam Jafri
|
|
|
Every day, human being faces serious challenges on how to co-operate with the newly added chemicals into his life. This includes chemicals in the drugs he takes, additives he eats in his food, pesticides in fruits and vegetables, pollutants from industrial factories, smoke from vehicles and other air particulates. These are only examples but not all we face every day of potential toxic hazardous and harmful teratogens. Teratogens are identified as substances that could induce malformations during embryonic development and they include chemicals (drugs, pesticides, petroleum, etc.), heavy metals, air pollutants, industrial wastes, contaminated water, viruses, stressors, and malnutrition. Only fractions of these agents are properly tested on their effects on embryonic development. This leads to the real needs of establishing in vivo and in vitro system to test existing and newly emerging teratogens.
|
Vision & Mission
Vision: To protect the Saudi Arabian Community from the early and late embryonic side effects of teratogens, toxins, air and water contaminants, pesticides, drugs and any other chemicals or environmental hazards.
Mission: To provide a robust and efficient aquatic and mammalian developmental teratogenicity and toxicity screening assay to the scientific research institutions as well as the industrial and public sectors.
Teratogenicity and Toxicity Application Assays
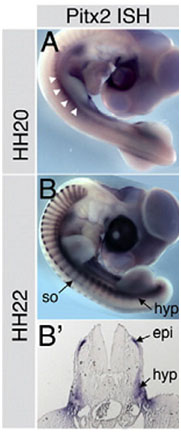
Three developmental teratogenicity and toxicity screening assays are offered, one using aquatic embryonic models (Zebrafish embryos), another using a vertebrate animal model (Chick embryo) and the third using a mammalian model using mouse embryos. We have the expertise to run the service professionally by an experienced developmental molecular biologist Dr. Muhammad Abu-Elmagd. To test an agent for its potential teratogenicity and toxicity side effects, embryos at different early and late stages will be exposed or administered to serial concentrations of the agent. After incubation period, embryos will be scored for lethality, toxicity and teratogenicity. Embryonic defects and phenotypes will be scored and documented based first on the morphological analysis and this will be followed by molecular analysis including changes in gene expression during organogenesis using in situ hybridization, microarray and/or alterations at the genomic level using next generation sequencing analysis. |
Teratogenicity and Toxicity Application Assays
 |
Electroporator |
 |
Injector |
 |
Needles’ puller |
 |
Zebrafish Aquaria (under construction) |
 |
Mouse breeding cages and facilities (KFMRC Animal House) |
 |
Hybridization oven |
 |
Affymetrix microarray facilities |
 |
NGS facilities |
|
| |
|